Localized Molecular Orbitals on:
[Wikipedia]
[Google]
[Amazon]
Localized molecular orbitals are
 Organic chemistry is often discussed in terms of localized molecular orbitals in a qualitative and informal sense. Historically, much of classical organic chemistry was built on the older
Organic chemistry is often discussed in terms of localized molecular orbitals in a qualitative and informal sense. Historically, much of classical organic chemistry was built on the older
molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of find ...
s which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock
In computational chemistry, post-Hartree–Fock methods are the set of methods developed to improve on the Hartree–Fock (HF), or self-consistent field (SCF) method. They add electron correlation which is a more accurate way of including the repu ...
electronic structure calculations by taking advantage of the local nature of electron correlation
Electronic correlation is the interaction between electrons in the electronic structure of a quantum system. The correlation energy is a measure of how much the movement of one electron is influenced by the presence of all other electrons.
Ato ...
. Localized orbitals in systems with periodic boundary conditions
Periodic boundary conditions (PBCs) are a set of boundary conditions which are often chosen for approximating a large (infinite) system by using a small part called a ''unit cell''. PBCs are often used in computer simulations and mathematical mod ...
are known as Wannier functions.
Standard ab initio quantum chemistry methods
''Ab initio'' quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excite ...
lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation
In mathematics, a unitary transformation is a transformation that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation.
Formal definition
More precisely, ...
.
In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The localized orbital corresponding to one O-H bond is the sum of these two delocalized orbitals, and the localized orbital for the other O-H bond is their difference; as per Valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
.
For multiple bonds and lone pairs, different localization procedures give different orbitals. The Boys and Edmiston-Ruedenberg localization methods mix these orbitals to give equivalent bent bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a ...
s in ethylene and ''rabbit ear'' lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
s in water, while the Pipek-Mezey method preserves their respective σ and π symmetry.
Equivalence of localized and delocalized orbital descriptions
For molecules with a closed electron shell, in which each molecular orbital is doubly occupied, the localized and delocalized orbital descriptions are in fact equivalent and represent the same physical state. It might seem, again using the example of water, that placing two electrons in the first bond and two ''other'' electrons in the second bond is not the same as having four electrons free to move over both bonds. However, in quantum mechanics all electrons are identical and cannot be distinguished as ''same'' or ''other''. The totalwavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ...
must have a form which satisfies the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formula ...
such as a Slater determinant
In quantum mechanics, a Slater determinant is an expression that describes the wave function of a multi-fermionic system. It satisfies anti-symmetry requirements, and consequently the Pauli principle, by changing sign upon exchange of two electro ...
(or linear combination of Slater determinants), and it can be shown that if two electrons are exchanged, such a function is unchanged by any unitary transformation of the doubly occupied orbitals.
For molecules with an open electron shell, in which some molecular orbitals are singly occupied, the electrons of alpha and beta spin must be localized separately. This applies to radical species such as nitric oxide and dioxygen. Again, in this case the localized and delocalized orbital descriptions are equivalent and represent the same physical state.
Computation methods
Localized molecular orbitals (LMO) are obtained byunitary transformation
In mathematics, a unitary transformation is a transformation that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation.
Formal definition
More precisely, ...
upon a set of canonical molecular orbitals (CMO). The transformation usually involves the optimization (either minimization or maximization) of the expectation value of a specific operator. The generic form of the localization potential is:
,
where is the localization operator and is a molecular spatial orbital. Many methodologies have been developed during the past decades, differing in the form of .
The optimization of the objective function is usually performed using pairwise Jacobi rotations. However, this approach is prone to saddle point convergence (if it even converges), and thus other approaches have also been developed, from simple conjugate gradient methods with exact line searches, to Newton-Raphson and trust-region methods.
Foster-Boys
The Foster-Boys (also known asBoys
A boy is a young male human. The term is commonly used for a child or an adolescent. When a male human reaches adulthood, he is described as a man.
Definition, etymology, and use
According to the ''Merriam-Webster Dictionary'', a boy is "a ...
) localization method minimizes the spatial extent of the orbitals by minimizing , where . This turns out to be equivalent to the easier task of maximizing . In one dimension, the Foster-Boys (FB) objective function can also be written as
.
Fourth moment
The fourth moment (FM) procedure is analogous to Foster-Boys scheme, however the orbital fourth moment is used instead of the orbital second moment. The objective function to be minimized is . The fourth moment method produces more localized virtual orbitals than Foster-Boys method, since it implies a larger penalty on the delocalized tails. For graphene (a delocalized system), the fourth moment method produces more localized occupied orbitals than Foster-Boys and Pipek-Mezey schemes.Edmiston-Ruedenberg
Edmiston-Ruedenberg localization maximizes the electronic self-repulsion energy by maximizing , where .Pipek-Mezey
Pipek-Mezey localization takes a slightly different approach, maximizing the sum of orbital-dependent partial charges on the nuclei: . Pipek and Mezey originally used Mulliken charges, which are mathematically ill defined. Recently, Pipek-Mezey style schemes based on a variety of mathematically well-defined partial charge estimates have been discussed. Some notable choices are Voronoi charges, Becke charges, Hirshfeld or Stockholder charges, intrinsic atomic orbital charges, Bader charges, or "fuzzy atom" charges. Rather surprisingly, despite of the wide variation in the (total) partial charges reproduced by the different estimates, analysis of the resulting Pipek-Mezey orbitals has shown that the localized orbitals are rather insensitive to the partial charge estimation scheme used in the localization process. However, due to the ill-defined mathematical nature of Mulliken charges (and Löwdin charges, which have also been used in some works), as better alternatives are nowadays available it is advisable to use them in favor of the original version. The most important quality of the Pipek-Mezey scheme is that it preserves σ-π separation in planar systems, which sets it apart from the Foster-Boys and Edmiston-Ruedenberg schemes that mix σ and π bonds. This property holds independent of the partial charge estimate used. While the usual formulation of the Pipek-Mezey method invokes an iterative procedure to localize the orbitals, a non-iterative method has also been recently suggested.In organic chemistry
 Organic chemistry is often discussed in terms of localized molecular orbitals in a qualitative and informal sense. Historically, much of classical organic chemistry was built on the older
Organic chemistry is often discussed in terms of localized molecular orbitals in a qualitative and informal sense. Historically, much of classical organic chemistry was built on the older valence bond
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
/ orbital hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to ...
models of bonding. To account for phenomena like aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
, this simple model of bonding is supplemented by semi-quantitative results from Hückel molecular orbital theory Hückel or Huckel may refer to:
* Erich Hückel (1896-1980), German physicist and chemist
** Debye–Hückel equation (named after Peter Debye and Erich Hückel), in chemistry, a method of calculating activity coefficients
** Hückel method (nam ...
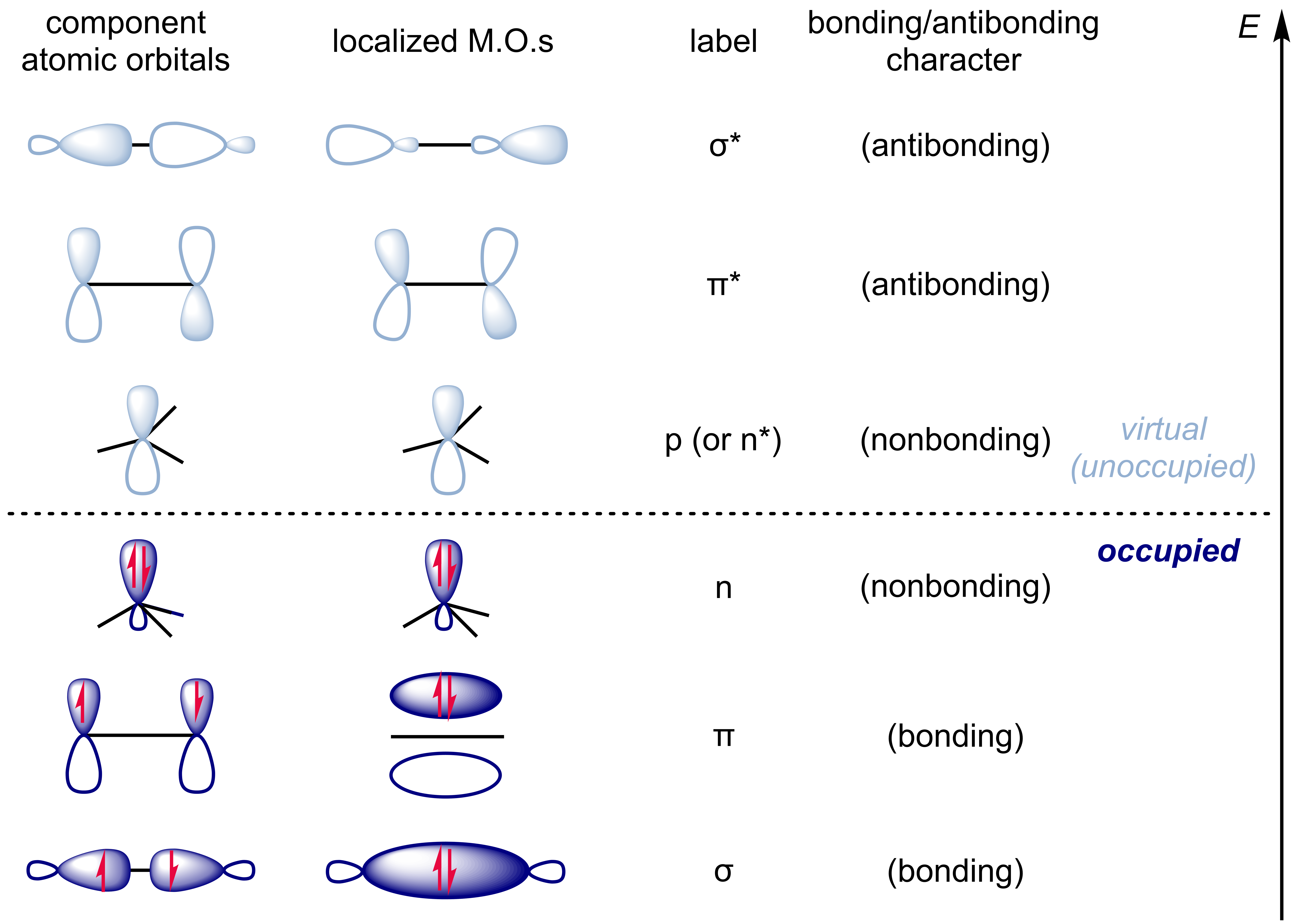
. However, the understanding of stereoelectronic effects requires the analysis of interactions between donor and acceptor orbitals between two molecules or different regions within the same molecule, and molecular orbitals must be considered. Because proper (symmetry-adapted) molecular orbitals are fully delocalized and do not admit a ready correspondence with the "bonds" of the molecule, as visualized by the practicing chemist, the most common approach is to instead consider the interaction between filled and unfilled localized molecular orbitals that correspond to σ bonds, π bonds, lone pairs, and their unoccupied counterparts. These orbitals and typically given the notation σ (sigma bonding), π (pi bonding), ''n'' (occupied nonbonding orbital, "lone pair"), ''p'' (unoccupied nonbonding orbital, "empty p orbital"; the symbol ''n''* for unoccupied nonbonding orbital is seldom used), π* (pi antibonding), and σ* (sigma antibonding). (Woodward and Hoffmann use ω for nonbonding orbitals in general, occupied or unoccupied.) When comparing localized molecular orbitals derived from the same atomic orbitals, these classes generally follow the order σ < π < ''n'' < ''p'' (''n''*) < π* < σ* when ranked by increasing energy.
The localized molecular orbitals that organic chemists often depict can be thought of as qualitative renderings of orbitals generated by the computational methods described above. However, they do not map onto any single approach, nor are they used consistently. For instance, the lone pairs of water are usually treated as two equivalent sp''x'' hybrid orbitals, while the corresponding "nonbonding" orbitals of carbenes
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carben ...
are generally treated as a filled σ(out) orbital and an unfilled pure p orbital, even though the lone pairs of water could be described analogously by filled σ(out) and p orbitals (''for further discussion, see the article on'' lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
''and the discussion above on'' sigma-pi and equivalent-orbital models The σ-π model and equivalent-orbital model refer to two possible representations of molecules in valence bond theory. The σ-π model differentiates bonds and lone pairs of σ symmetry from those of π symmetry, while the equivalent-orbital model ...
). In other words, the type of localized orbital invoked depends on context and considerations of convenience and utility.
References